MICHAEL SCHAEFER
Safety Risk Management
Simplicity and ease-of-use in Risk Management
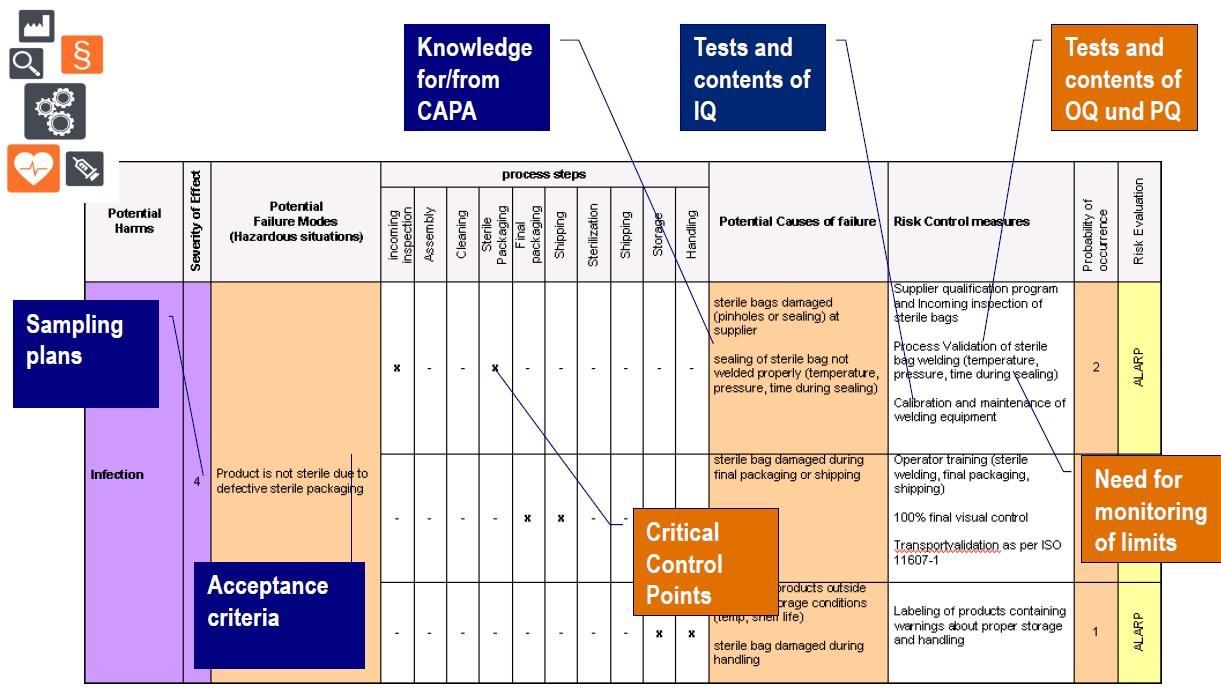
Medical device industry invested a considerable amount of time and efforts to generate and maintain risk management documentation as per ISO 14971. Often it was mainly done to satisfy the expectations from authorities or notified bodies. We should now be ready for the next step, to turn these investments into value adding tools and to make them easier-to-use for engineers.
The materials provided on this page will show how to simplify risk management and how to use the information more effectively while carefully avoiding overloaded and non-sustainable quality systems. The attached templates may be used and trainings are available including workshops.
Harms and severities – applying IMDRF and CTCAE
Manufacturers sometimes struggle to determine adequate medical terms and to assign reasonable severity ratings as required by ISO 14971. In the past there was very limited guidance available discussing this task. In the meanwhile there were some helpful documents published making the challenge a bit easier and much more transparent. I want to remind you of IMDRF guidance on terminologies for adverse events and CTCAE Common Terminology Criteria for Adverse Events. Both fit together really well, see my article here.
Risk management for suppliers
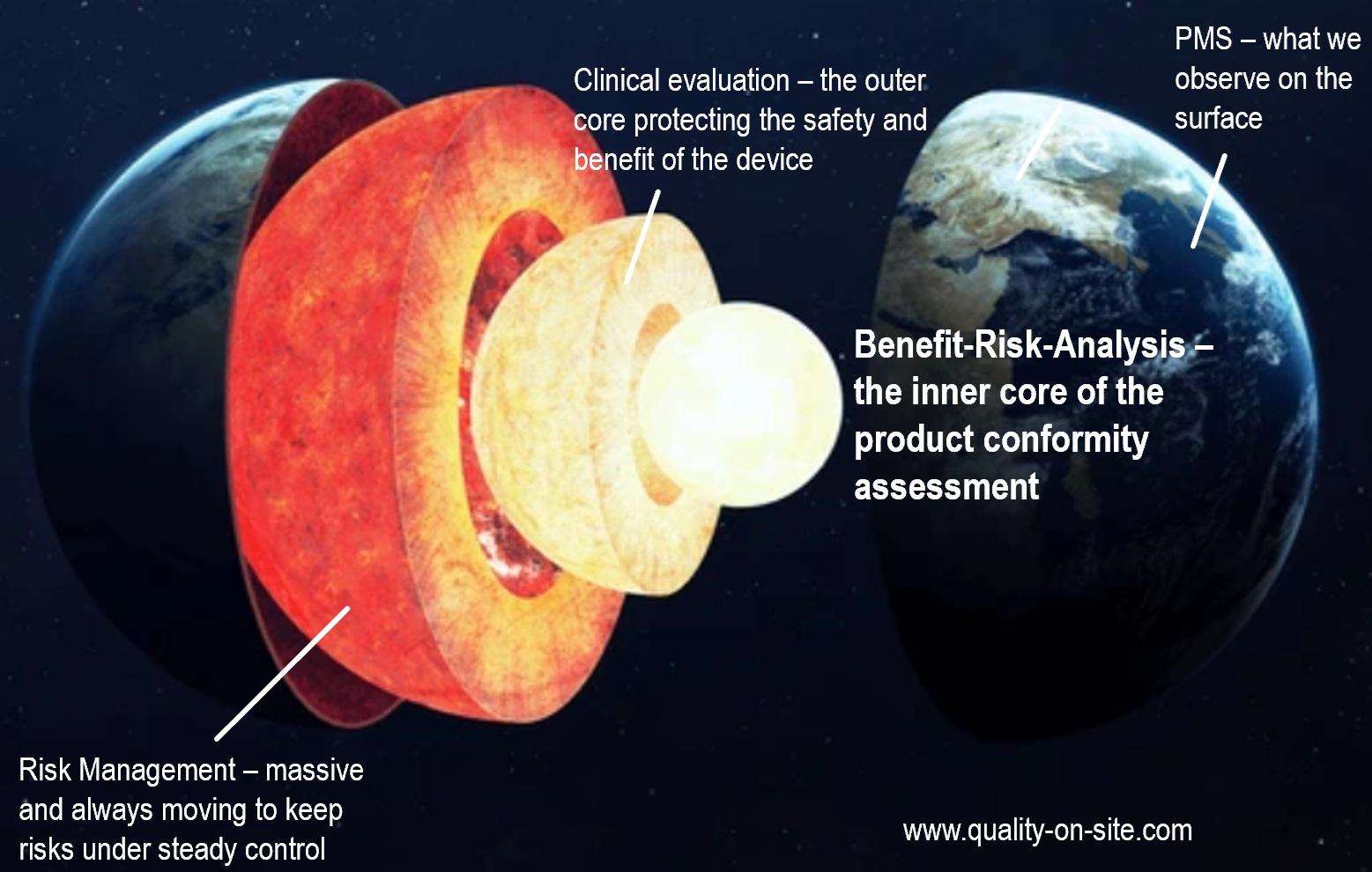
Safety risk management as per ISO 14971:2019 aims to protect patients and users from harm caused by medical devices. Over the last two decades, medical device manufactures got used to this standard and are more or less compliant to its requirements. However, many supplier are challenged by implementing the requirements of ISO 14971 in their quality systems. Being scrutinized by customers and/or certification bodies, suppliers are struggling to get access to the medical and technical information needed to conduct proper risk management. Consequently, not knowing the details about the technologies and usage of the final medical devices, not having access to the PMS data needed and not being involved in the clinical evaluation and benefits, suppliers often implement insufficient risk management documentation just for the sake of satisfying their customers and their auditors. It is obvious, that such situation unfortunately leads to frustration and a limited acceptance of risk management principles. A supplemental risk management plan for suppliers may help adressing this issue.
Downloads:
Template Risk Analysis (FTA type)
Template Risk Analysis (HA-FMEA type)
Template Risk Management Report
Address
Heiligkreuzstrasse 59,
72379 Hechingen Germany