MICHAEL SCHAEFER
Regulatory Affairs and Quality Management
On this page I am diving into some topics in the huge area of regulatory affairs and quality management. For each topic you will find some information for download.
Technical documentation as per MDR
Within MDR, the Technical Documentation was structured as per GHTF STED and is now described in Annex II of the new MDR. You can download a free example for a table of content of a technical documentation for a non-active medical device. For purpose of international registrations, some additional requirements were added.
The formerly 13 Essential Requirements of MDD were replaced by 23 General Safety and Performance Requirements. Unfortunately, the MDR doesn’t provide a trace matrix between the current and the new requirements. The file that can be downloaded here was compiled to provide such comparison. Although it was actually quite difficult to align the new requirements one-by-one with the current requirements, the table may be supportive when manufacturers are asked to establish a revised checklist for the conformity assessment under the new MDR.
Medical device manufacturers acting globally should decide carefully about the structure of their technical documentation. Setting up the right format will prevent redundant documentation and non-value adding expenses. The document attached gives a generic overview of STED, MDR and ToC formats. It can be seen, that all three approaches are pretty equivalent. For sure, differences can be found when diving deeper into details and requirements.
Economic operators
MDR introduced the concept of the Economic Operators. An organization shall identify their roles undertaken in Europe. These roles can include Manufacturer, Authorized representative, Importer or Distributor. These terms were already introduced within the ISO 13485:2016. Articles 10 to 14 of MDR define the obligations of these Economic Operators. The attached document provides a rough and generic overview of these obligations.
Basic UDI-DI
The European Union introduced the UDI for medical devices as per MDR 2017/745. When looking into the details one may not expect too many differences versus the UDI introduced recently by the US FDA. However, some differences exist. Besides the main difference, that EU will use the EUDAMED database and US will use their GUDID database, another main difference is the “Basic UDI-DI”. The Basic UDI-DI is defined by MDR as “the primary identifier of a device model. It is the main key for records in the UDI database and is referenced in relevant certificates and EU declarations of conformity” [MDR2017/745, Annex VI, Part C]. The Basic UDI-DI is to be seen as the access key to EUDAMED, i.e. the database where all UDI information will be held. It is important to know, that the Basic UDI-DI is different to the UDI-DI itself. The Basic UDI-DI will not be labelled on the packaging and will not be used in supply chain. It is more like a regulatory identifier and will be used in the Declaration of Conformity, Technical Documentation, Free Sales certificates and in the SSCP. The UDI-DI is to be included in the labelling and in EUDAMED. There are some more differences between the EU and US UDI. Active implants will have to bear the individual serial number in the UDI-PI in Europe, which is currently not required by FDA. The date format is a given in US (YYYY-MM-DD) whereas it is not defined in Europe. Some more differences, mainly product specific, can be found when digging into Annex VI of the MDR.
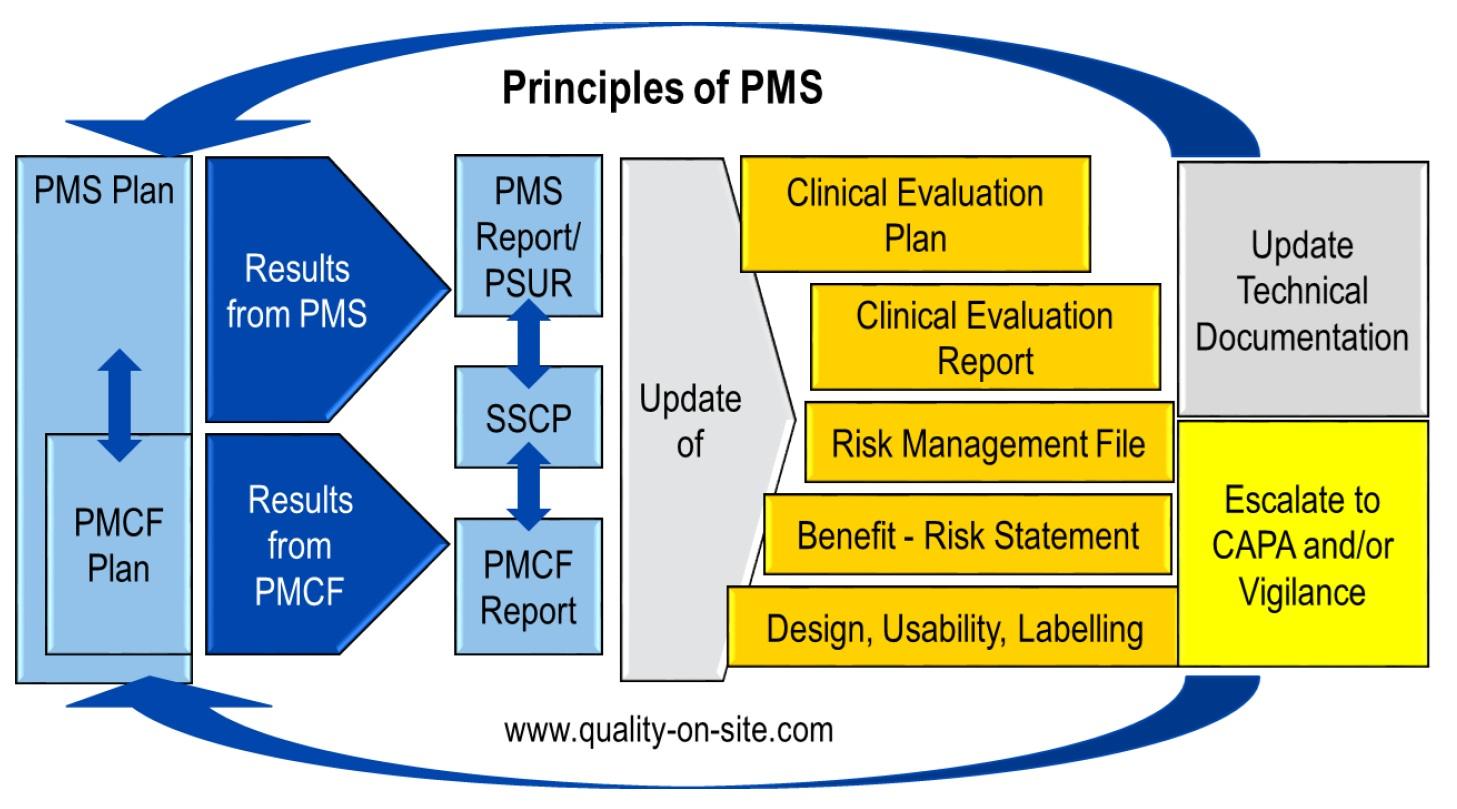
SOP Post-Market Surveillance (PMS)
MDR reminder: As per article 83 of MDR, manufacturers must establish a comprehensive post-market-surveillance System under their quality management system. Keep in mind, that ALL manufacturers must fulfill article 83 after May 2021, both under MDD and MDR! However, many manufacturers struggle to get this task accomplished. You will find therefore a SOP PMS on my webpage for download. Feel free to use it, but please provide feedback about the experience you made using it. I also developed a template for a PMCF plan, feel free to use it!
Change control management
Changes to design, manufacturing and QMS must be documented and evaluated. The checklist changes (Checkliste Änderungsantrag) will support such activities. Keep in mind, that article 120.3 of MDR may be applicable as well.
Quality policy and quality objectives
Some things can be kept simple in our quality systems and manufacturers struggle to find the right level for quality policy and quality objectives. Therefore I came up with a simple document guiding you to a smart and compliant solution.
Supplier Qualification
One topic still causing major headache is the classification of suppliers. A Babylon of misunderstandings, different opinions from notified bodies and the MDSAP interpretation made it difficult in the past to come-up with an easy way of classifying suppliers. Within the attached flowchart for supplier qualification I will treat suppliers, service providers and outsourced processes all the same (as indicated by GHTF/SG3/N17R9:2008) and cover MDSAP terminology as well. Still the flowchart offers an easy way to classify suppliers and helps to determine the requirements for qualification.
DOWNLOADS:
Template Plan for Post-Market Clinical Follow-Up
Template Checkliste Aenderungsantrag
Template Checkliste Article 120
Address
Heiligkreuzstrasse 59,
72379 Hechingen Germany